
For example the mass number of a regular carbon atom is 12, since a carbon atom has 6 protons and 6 neutrons in its nuclus.
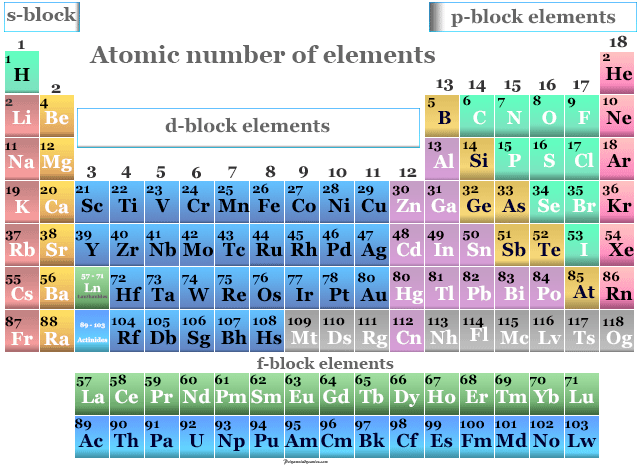
Periodic table with atomic number and atomic mass plus#
In words, the mass number is the number of neutrons in an atom of a specific element plus the number of protons in an atom of that element. Isotopes are forms of elements that have the same number of protons and therefore the same atomic number, but a different number of neutrons which affects their mass number. Mass NumberĪll atoms have a mass number which is derived as follows: The atomic number of an element never changes, meaning that the number of protons in the nucleus of every atom in an element is always the same. Oxygen atoms contain 8 protons and have an atomic number of 8. All carbon atoms, and only carbon atoms, contain six protons and have an atomic number of 6. For example, all hydrogen atoms, and only hydrogen atoms, contain one proton and have an atomic number of 1. In other words, each element has a unique number that identifies how many protons are in one atom of that element. The number of protons in the nucleus of an atom determines an element's atomic number. Define and determine the mass number of an atom.
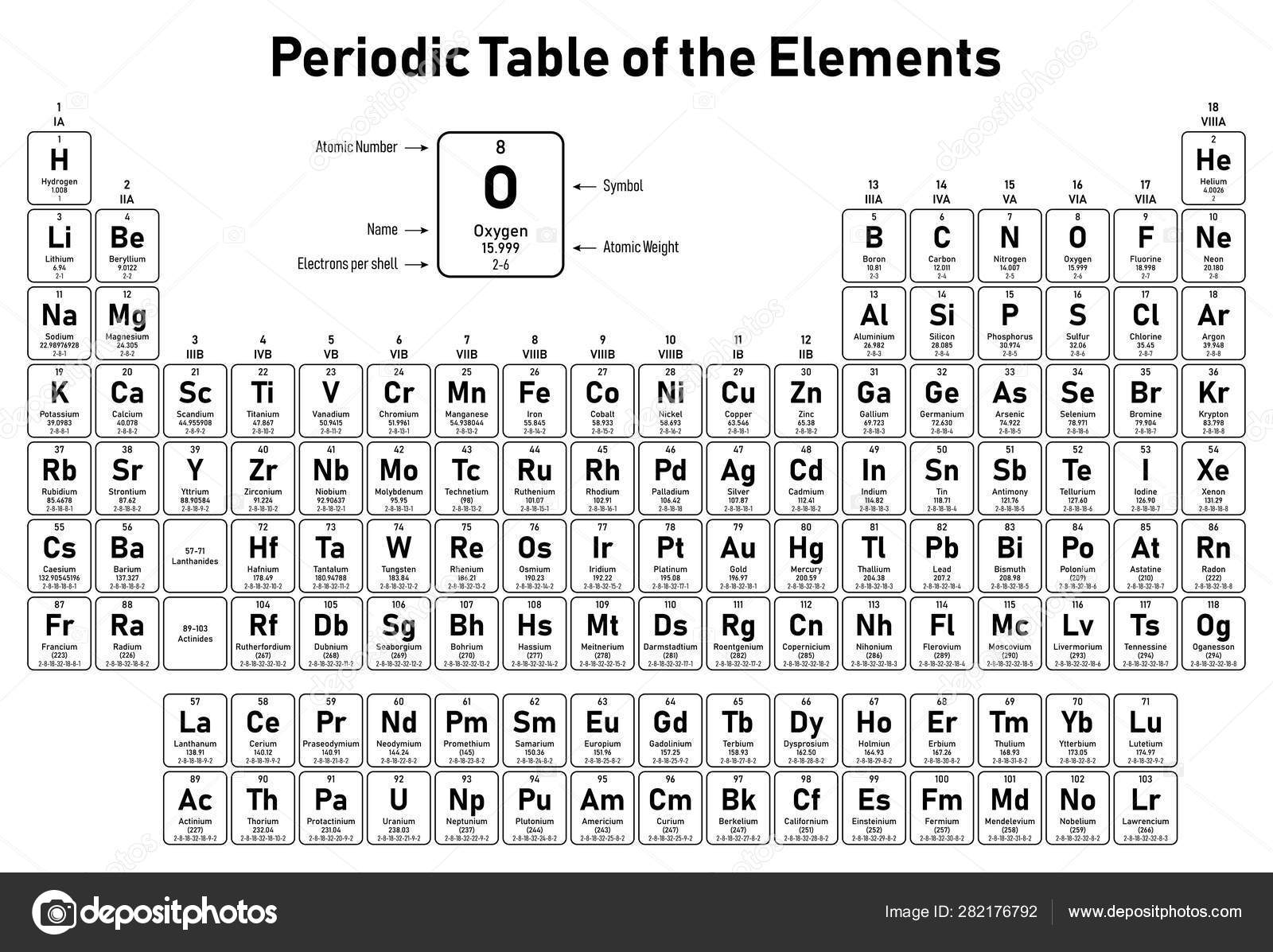
Define and determine the atomic number of an atom.After reading this section you will be able to do the following:


 0 kommentar(er)
0 kommentar(er)
